The US wet wipes manufacturers market is projected to reach USD 8.54 billion by 2030 (grandviewresearch.com), creating intense competition for brands seeking reliable manufacturing partners. Many companies struggle to find private label and contract wet wipe manufacturers that deliver custom formulations, airtight regulatory compliance, and eco-friendly materials. This guide unpacks how private label services, contract production, step-by-step manufacturing, sustainable practices, regulatory requirements, quality assurance, and emerging market trends work together to build topical authority in wet wipe manufacturing.
In the sections that follow, you will discover:
1. The mechanics of contract wet wipe manufacturing in US facilities
2. A detailed manufacturing process from raw materials to finished packs
3. Sustainable production techniques reshaping the industry
4. Key regulatory frameworks from FDA, EPA and ISO standards
5. Advanced quality assurance measures that ensure consistent performance
6. The latest market trends and innovations driving new applications
This roadmap aligns all aspects of wet wipe manufacturing to empower brand owners and guide informed decisions.
What Is Private Label Wet Wipe Manufacturing?
Private label wet wipe manufacturing refers to the service where a specialized production partner handles the entire manufacturing lifecycle—from nonwoven substrate selection and solution formulation to cutting, folding, and packaging—under a client’s branding specifications. This arrangement reduces capital expenditure, minimizes lead times, and allows for tight control over product attributes like moisture level and gentle ingredients.
How Does Custom Wet Wipe Formulation Support Brand Differentiation?
Custom formulation in wet wipe manufacturing enables brands to introduce unique features—such as alcohol-free sanitizing solutions, skin-soothing botanicals, or pH-balanced blends—that set products apart and address niche markets. For example, integrating aloe vera and chamomile extracts enhances skin comfort, while antimicrobial peptides boost disinfectant efficacy. These specialized solutions create compelling selling points that foster brand loyalty.


What Packaging Options Are Available for Private Label Wet Wipes?
Manufacturers typically offer a range of packaging formats to match usage scenarios and shelf appeal.

Each packaging choice influences consumer experience and shelf-life stability, guiding brand owners to align format with end-use scenarios and sustainability objectives.
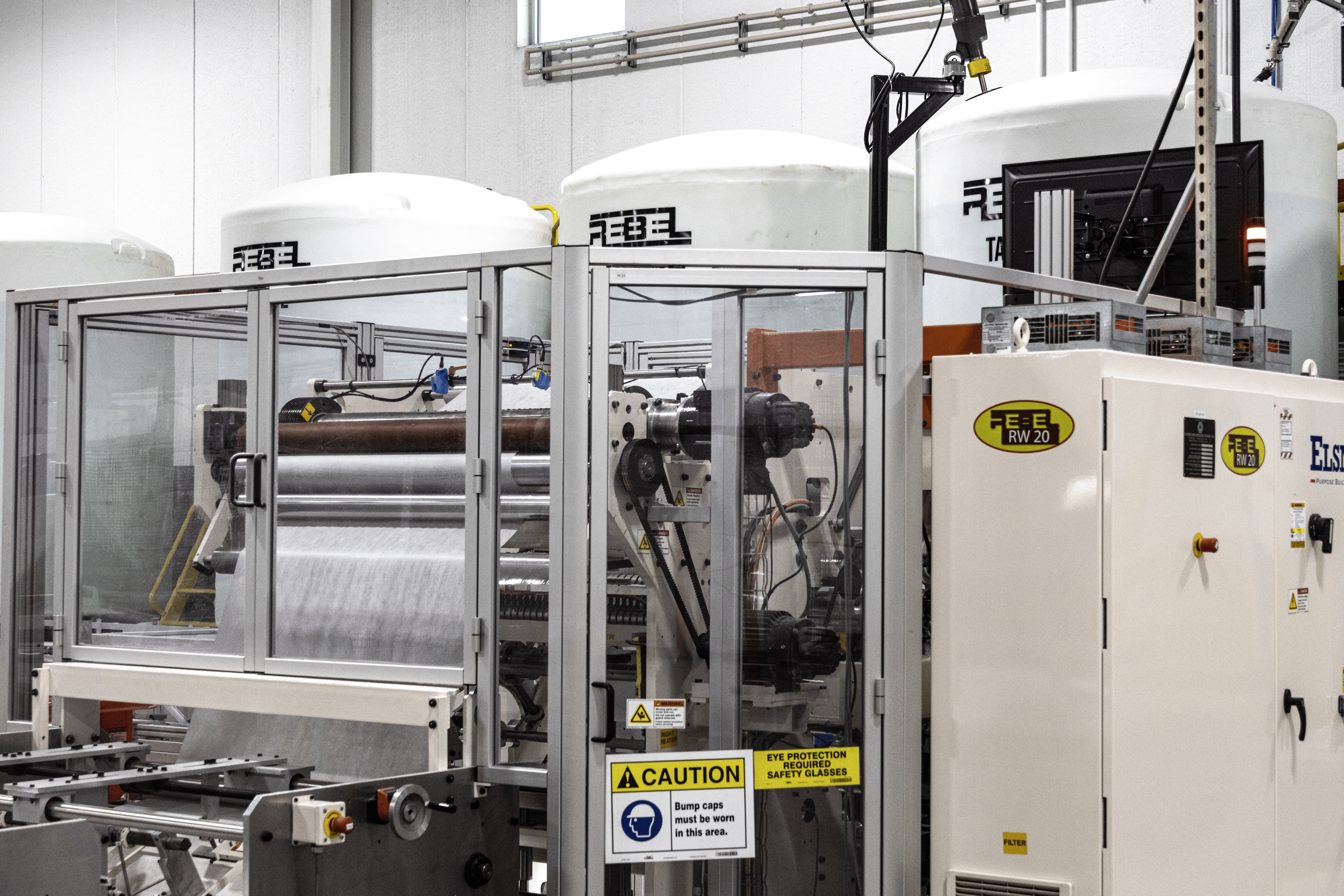
How Does Contract Wet Wipe Manufacturing Work in the US?
Contract wet wipe manufacturing allows brands to outsource full production workflows to specialized facilities such as Rebel Converting in the US, combining scalable capacity, advanced cleanroom environments, and rigorous quality systems to deliver consistent, compliant products under a partnership model.
What Are the Key Benefits of Contract Wet Wipe Manufacturing?
Contract services offer several strategic advantages:
● Scalability: Access to high-volume converting lines without capital investment
● Cost Efficiency: Economies of scale reduce per-unit costs on raw materials and labor
● Regulatory Compliance: Established protocols for FDA, EPA, and ISO standards
● Speed to Market: Ready-to-use production slots accelerate launch timelines
Contract manufacturing offers a strategic approach for brands to enter or expand in the wet wipe market.
Which Production Stages Are Included in Contract Manufacturing Services?

Contract providers typically manage five core stages:
1. Raw Material Sourcing – Procurement of nonwoven fabric rolls, purified water, chemicals.
2. Solution Preparation – Formulating liquid blends with preservatives, surfactants, and actives.
3. Converting – Cutting rolls to size and folding substrates into wipe format.
4. Impregnation – Saturating substrate with solution under controlled tension.
5. Packaging & Labeling – Sealing wipes into chosen formats with tamper-evident closures.
This end-to-end coverage ensures brands can focus on sales while outsourcing technical complexities.
How Does Supply Chain Management Impact Contract Wet Wipe Production?
A resilient supply chain safeguards continuous production by diversifying raw material suppliers, maintaining buffer inventories, and tracking lead times for critical inputs. For instance, sourcing nonwoven fabrics from multiple domestic mills reduces disruption risk, while just-in-time logistics for chemicals optimize warehouse utilization without compromising run rates.
What Are the Differences Between OEM and Contract Manufacturing for Wet Wipes?
While both OEM and contract manufacturing involve third-party production, the distinctions lie in customization and branding:
● OEM (Original Equipment Manufacturer): Produces goods to the client’s complete design, often sold under the manufacturer’s brand or generic labels.
● Contract Manufacturing: Rebel Converting produces and labels products exclusively for the contracting brand, offering deeper collaboration on formulation, packaging, and private labeling.
Brands seeking full control over branding and product identity generally prefer contract manufacturing partnerships.
What Is the Step-by-Step Wet Wipe Manufacturing Process?
The wet wipe manufacturing process integrates specialized equipment and quality controls to transform raw materials into finished, ready-to-use products.
Which Raw Materials Are Used in Wet Wipe Production?
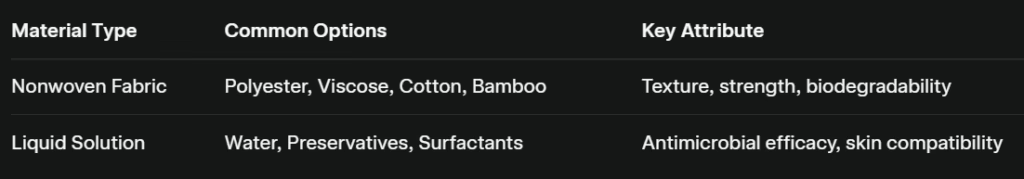
Selecting the proper fabric blend ensures the wipe’s tensile strength, softness, and absorption characteristics align with its intended use.
How Are Wet Wipes Cut, Folded, and Impregnated?
During the converting stage, large rolls are slit to precise widths, then folded by automated machinery into single-layer or multi-layer configurations. Impregnation machines then uniformly saturate each wipe with the liquid blend under controlled tension, ensuring consistent moisture content across every sheet.
What Packaging Methods Ensure Wet Wipe Quality and Convenience?
Packaging preserves moisture, ease of dispensing, and consumer convenience:
● Soft-packs maintain seal integrity via resealable flaps.
● Canisters support larger wipe counts and protect against deformation.
● Flow-wrap sachets provide single-use hygiene solutions.
Packaging design directly impacts shelf life, user satisfaction, and brand perception.
How Is Quality Control Implemented Throughout Manufacturing?
Quality assurance is embedded at each stage through in-line and laboratory testing:
● In-Line Checks monitor fabric uniformity, moisture levels, and tension.
● Raw Material Inspections verify fiber composition and solution pH.
● Finished Product Tests assess tear strength, microbial load, and preservative efficacy.
These controls underpin consistent product performance and compliance with industry standards.
How Is Sustainable Wet Wipe Production Shaping the Industry?
Sustainable wet wipe production integrates biodegradable materials, eco-friendly formulations, and green manufacturing practices to reduce environmental impact from raw material sourcing to end-of-life disposal.
The industry is shifting towards sustainable practices to meet consumer demand and reduce environmental impact.
What Biodegradable Materials Are Used in Eco-Friendly Wet Wipes?
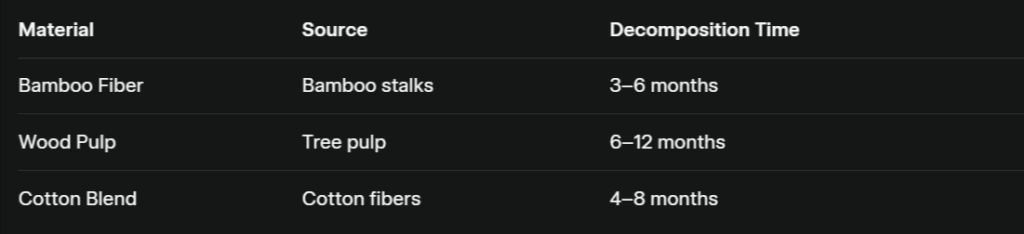
These substrates offer comparable strength and softness while minimizing landfill persistence. (raan.com)
How Are Sustainable Formulations Developed for Wet Wipes?



Eco-friendly formulations replace petrochemical preservatives and surfactants with plant-derived alternatives—such as grapefruit seed extract, decyl glucoside, and citric acid buffers—maintaining antimicrobial performance without harsh residues.
What Packaging Solutions Reduce Environmental Impact?
Brands adopt compostable or recyclable packaging films and water-soluble labels to align with circular economy goals. Post-consumer take-back programs and refill pouch options further cut plastic usage and logistics emissions.
How Does Sustainable Manufacturing Reduce Carbon Footprint?
Green production practices include solar-powered facilities, on-site water recycling for formulation mixing, and optimized logistics routing. Together, these initiatives can cut greenhouse gas emissions by up to 30 percent compared to conventional production models.(sciencedirect.com)
What Are the Regulatory Requirements for Wet Wipe Manufacturers in the US?
Wet wipe manufacturers must navigate overlapping regulations from the FDA, EPA, and international quality standards to ensure product safety, efficacy, and legal compliance.
How Does the FDA Regulate Wet Wipes as Cosmetics, Drugs, or Medical Devices?
Under FDA authority, wet wipes may be classified as:
● Cosmetics if intended for cleansing, fragrancing, or beautifying without therapeutic claims.
● Over-the-Counter Drugs if bearing antimicrobial or antiseptic claims.
● Medical Devices when designed for specific clinical or diagnostic purposes.
Each category demands distinct registration, labeling, and safety assessments to validate product claims.
The FDA’s classification determines the regulatory requirements for wet wipe manufacturers.
What EPA Regulations Apply to Disinfectant Wet Wipes?
Disinfectant wipes require EPA registration as antimicrobial pesticides, including:
1. Submission of efficacy data against specified pathogens.
2. Compliance with residual risk assessments and labeling limits.
3. Adherence to Worker Protection Standards for manufacturing staff.
Rigorous testing protocols ensure wipe efficacy without compromising user or environmental safety.
EPA regulations ensure the safety and efficacy of disinfectant wipes.
How Do ISO Certifications Like ISO 13485 and GMP Ensure Product Safety?
Implementing ISO 13485 (medical devices) and Good Manufacturing Practices (GMP) under ISO 22716 provides a structured quality management system covering documentation, process controls, and facility hygiene. These standards reduce risk, support audit readiness, and bolster customer confidence.
ISO certifications demonstrate adherence to international best practices and ensure consistent quality.
What Are Common Compliance Challenges and How Does Rebel Converting Address Them?
Manufacturers often face shifting preservative regulations, complex labeling requirements, and audit preparedness. Rebel Converting addresses these challenges by maintaining a dedicated compliance team that continuously monitors regulatory updates, conducts internal mock audits, and designs documentation templates aligned with FDA, EPA, and ISO specifications.
How Is Quality Assurance Maintained in Wet Wipe Manufacturing?
Quality assurance in wet wipe production combines real-time monitoring, raw material verification, finished product testing, and third-party audits to uphold stringent performance standards.
What In-Line Quality Control Measures Are Used During Production?
In-line systems utilize optical sensors and moisture gauges to detect fabric imperfections, measure solution pickup, and adjust machine parameters on the fly to prevent defects and ensure uniformity.
How Are Raw Materials Tested for Consistency and Safety?
Material quality labs perform fiber content analysis, microbial screening, and solution pH tests to confirm that incoming fabrics and liquid blends meet predefined safety and performance criteria.
What Finished Product Tests Ensure Wet Wipe Performance?
Finished wipes undergo a series of evaluations:
● Tensile Strength tests for durability under tension.
● Moisture Content Analysis verifies target saturation levels.
● Microbial Challenge Tests confirm preservative efficacy.
These assessments guarantee the wipe’s robustness, safety, and usability.
Which Certifications and Audits Validate Quality Assurance?
Manufacturers seek certifications such as ISO 9001 (quality management), ISO 13485 (medical devices), and third-party audits (e.g., UL, NSF) to demonstrate adherence to international best practices and reassure customers of consistent quality.
What Are the Latest Market Trends and Innovations in Wet Wipe Manufacturing?

Rapidly evolving consumer needs and technological advances are driving new wet wipe applications, materials, and production methods that enhance functionality and environmental performance.
These trends are shaping the future of the wet wipe industry.
How Is Innovation Driving New Wet Wipe Formulations and Materials?
R&D efforts focus on embedding active textiles (e.g., slow-release antimicrobial fibers), nanofiber membranes for controlled solution release, and bio-based surfactants to improve cleaning power without skin irritation.
What Are Emerging Niche Applications for Wet Wipes?
Specialty segments are expanding to include:
● Cleanroom Wipes for electronics and pharmaceutical manufacturing
● Pet Grooming Wipes infused with conditioning agents
● Medical Device Wipes treated with hospital-grade disinfectants
These niche markets require tailored substrate and solution combinations to meet specific performance and regulatory demands.
How Are Consumer Preferences Influencing Product Development?
Consumers now demand multifunctional wipes—combining cleansing, moisturizing, and sanitizing in a single sheet—and favor plant-based, hypoallergenic ingredients. Brands respond by formulating hybrid solutions that address multiple needs in one application.
What Supply Chain Strategies Enhance Manufacturing Resilience?

Proactive inventory buffering, multi-vendor sourcing, and digital tracking systems enable manufacturers to adapt quickly to material shortages, regional disruptions, or sudden demand surges, ensuring uninterrupted production and delivery.
Despite the complexity of private label, contract, and sustainable wet wipe production, brands can navigate each stage—from formulation and packaging to compliance and quality assurance—by partnering with an experienced manufacturer. Rebel Converting combines advanced clean facilities, seasoned regulatory expertise, and a commitment to eco-friendly materials. Whether scaling disinfectant wipes, or pioneering bio-based substrates, this guide offers the foundation for informed decision-making and accelerated market entry. For tailored manufacturing solutions that blend innovation with reliability, explore how Rebel Converting can transform your wet wipe vision into high-quality products that resonate with today’s consumers.
Was this guide useful to you? Feel free to reach out to Rebel Converting with any questions at all.




0 Comments